MiFC Aims:
1) Offer a unique avenue to explore mitochondrial function and aid in the advancement of clinically relevant research.
2) Facilitate interdisciplinary collaboration in the field of metabolic and mitochondrial disease.
3) Provide accurate and sensitive high resolution analysis of respiratory capacity in biological samples including isolated mitochondria, living and permeabilized cells, and permeabilized in-situ tissue samples (heart, kidney, brain, liver, fat, skeletal muscle).
Core Capabilities

Our core is equipped with one Oroboros O2K High Resolution Respirometer as well as the latest version of DatLab (7.4). High Resolution Respirometry (HRR) allows for the state-of-the-art measurement of mitochondrial function in a variety of sample preparations. The Mitochondrial Function Core (MiFC) can accurately assess mitochondrial functional capacity using versatile substrate-uncoupler-inhibitor-titration (SUIT) protocols allowing for the analysis of a variety of mitochondrial pathways and states (LEAK, OXPHOS, ETS Capacity) in a single assay. Additionally, the Oroboros O2K offers an advantage over conventional mitochondrial function assays such as the seahorse, due to its ability to assess mitochondrial function in isolated mitochondria and live cells, as well as permeabilized cells and a variety of tissues (Heart, Liver, Fat, Kidney, Brain, Skeletal Muscle) using mg amounts. The O2K allows for respirometric measurements while maintaining the structural integrity of the mitochondrial network in cells and tissue, a prominent contributor to tissue metabolic function.
Additionally, using our TIP2K module we can test mitochondrial function in real time during the well controlled titration of drugs into a sample. Our O2k-Fluo Smart-Module optical sensors also allows us to measure hydrogen peroxide production (Amplex UltraRed), ATP production (Magnesium green), mt-membrane potential (Safranin, TMRM), and Ca2+ (Calcium green) during the simultaneous measurement of respirometry in a variety of states and pathways.
Sample Experiment
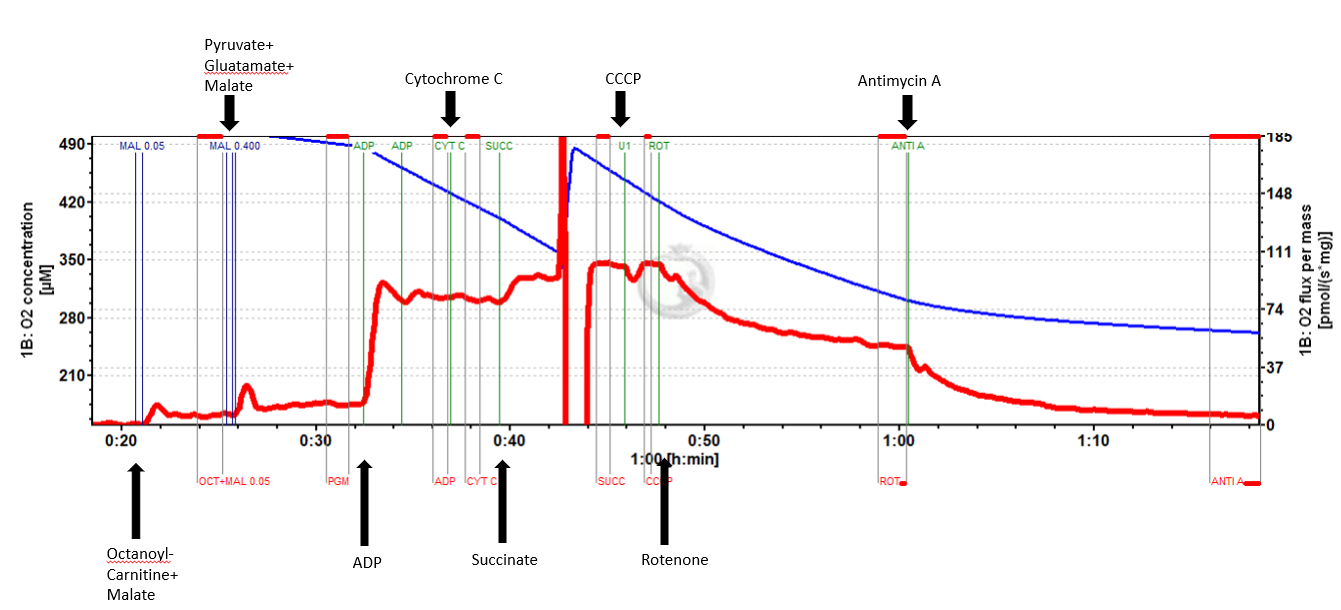
In short, mouse tibialis anterior was excised from a 33 month old mouse. Two 5 mg portions of the medial tibialis anterior were cut out and placed in preservation buffer (BIOPS). After mechanical separation and saponin permeabilization, each 5 mg bundle was placed into one chamber of the O2K containing mitochondrial respiration buffer Mir05. After signal stabilization, the following suit protocol was applied:
*Values for each tissue sample replicate were averaged
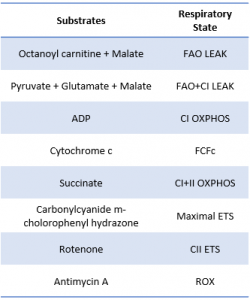
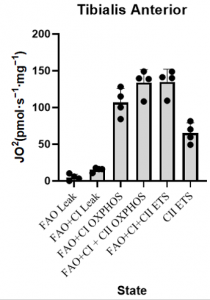
*This SUIT protocol allowed for measurement of LEAK supported by fatty acid oxidation (FAO) as well as leak supported by FAO+ Complex I (CI) substrates (pyruvate and glutamate). Sequential titration of kinetically saturating ADP allowed for measurement of oxidative phosphorylation capacity supported by electron input through FAO and CI. Cytochrome C titration following ADP is used as a test of sample integrity. Addition of succinate allowed for measurement of OXPHOS supported by convergent electron input from FAO, CI, and complex II (CII) of the mitochondrial electron transport system (ETS). CCCP titration is used to measure maximal FAO+CI+CII ETS capacity, followed by rotenone to inhibit complex I and measure CII supported ETS capacity. Lastly Antimycin A is titrated to gain a measure of residual oxygen consumption (ROX) to normalize all other values to.
Please contact us for collaborations for your mitochondrial respiration experiments
